About Sources for NDIR Gas Sensors
Nondispersive infrared (NDIR) spectroscopy is often used to detect gas such as carbon dioxide, nitrogen dioxide, sulfur dioxide, etc., and measure the concentration levels. In high concentrations these gasses can be lethal or explosive, making the monitoring for these gasses imperative in certain environments.
The technique is said to be nondispersive because no dispersive elements (e.g a prism or diffraction grating) are used and the wavelength that passes through the sampling chamber is not pre-filtered.
The majority of NDIR sensors use a broadband light source, a gas chamber with highly reflective walls, optical filter(s), detector(s), and electronics for signal processing. Absorption of infrared light occurs at the IR energy (i.e. wavelength) specific to the target gas, which means it is high selectivity to a specific gas which is key to NDIR gas detection
How Sources for NDIR Gas Sensors & Analyzers Work:
When infrared radiation interacts with gas molecules, infrared light is absorbed by the gas molecules at a specific band or wavelength, causing vibration of the gas molecules and absorption. The ratio of transmitted radiation energy to the incident energy reaching the detector, is dependent on target gas concentration. NDIR (Non-Dispersive Infrared) gas sensors detect the decrease in transmitted infrared light which is in proportion to the gas concentration.
Single vs Dual sensing gas sensing designs
The level of infrared light that the detector receives plays an important role in sensor drift and measurement accuracy. The infrared light source will have degradation of illumination over time from film buildup and age. There are also environmental factors to consider. The effects of temperature on an NDIR sensor are complex and care must be taken as changes in temperature reflect the absorbance, SPAN and apparent gas concentration.
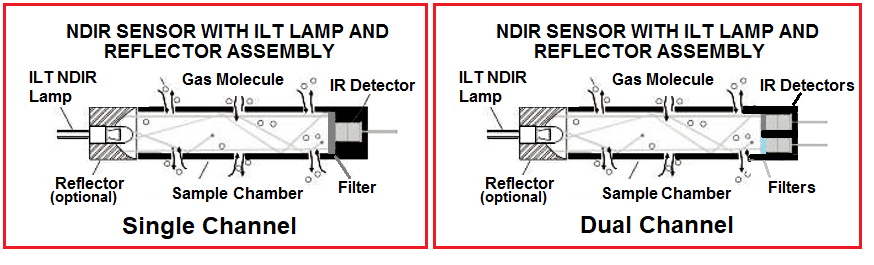
Single channel NDIR sensors often use electronics with complex algorithms for temperature compensation. The thermopile (IR) detector senses temperature by absorbing radiation, but it also responds to changes in ambient temperature which can give rise to spurious and inaccurate signals. For this reason, many NDIR sensors use a thermistor integrated into the package or use a dual wavelength sensing configuration to create a reference and incident light level comparison.
In the single sensor NDIR application thermistors are often used over temperature sensors. Pulsed and filtered IR light is applied to the series connected active junctions. The junctions are heated, which in turn generates a small thermoelectric voltage. The temperature of the reference junction is measured with a thermistor. The potential at the junction between the resistors can then be used to determine the thermistor resistance and the temperature. Use of a the thermistor in the sensor is more accurate than testing ambient temperature because the NDIR sensor will be slightly heated (often as much as 2 – 5 degrees) by the IR light source.
Dual wavelength type gas sensors use one light source, but have two detectors and two optical filters of different wavelengths which are placed in front of each detector. The reference wavelength selected is one that is unaffected by the sensed gas. As the infrared light source changes over time, the gas measurement can be compensated accordingly, eliminating sensor drift errors. In this way changes to lamp output (due to aging, temperature or other ambient conditions) can be accounted for and eliminated from the results.
Gas Sensing - By Gas Type
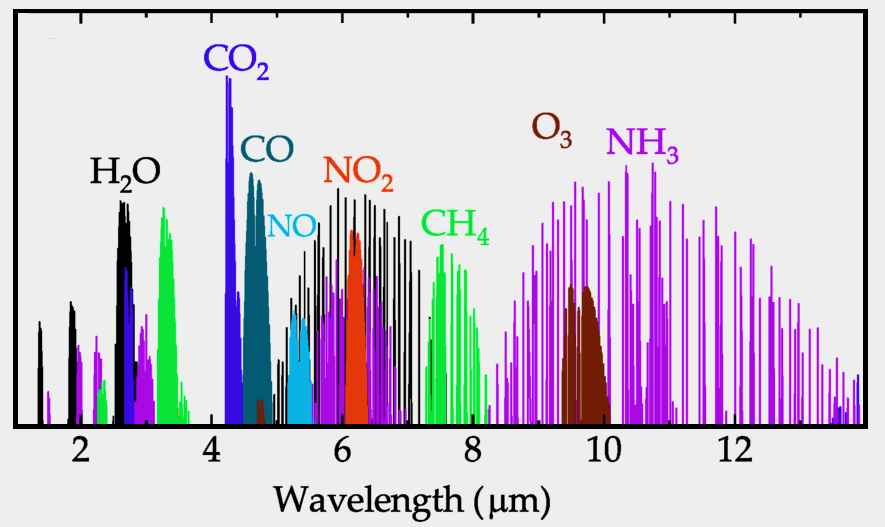
Carbon Dioxide (CO2) carbon dioxide is a by-product of making fertilizer and burning fuels. &Increased levels of CO2 cause discomfort followed by various physical issues such as headache, drowsiness, and fatigue. It also causes declines in productivity as well as effecting affect sleep. NDIR sensors for carbon dioxide are often encountered in heating, ventilation, and air conditioning (HVAC) units and can be used to adjust ventilation for the space. Carbon dioxide has a very strong absorption band between 4200 nm and 4300 nm.
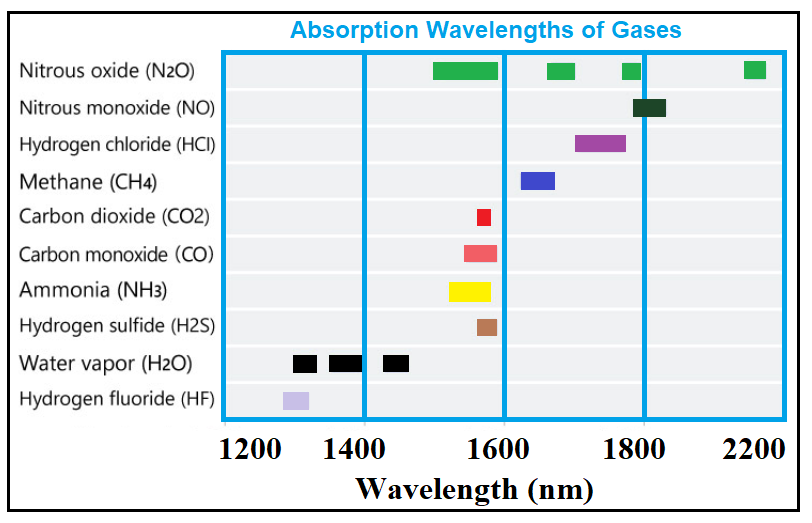
Carbon Monoxide (CO) is a tasteless color gas of which high levels are commonly found in populated areas generated by the burning of fossil fuels in vehicles and heating systems. Packaged carbon monoxide is used in a variety of industries for a wide range of applications including metal and chemical fabrication. Referred to as ‘the silent killer’ is still responsible for tens of thousands of deaths each year. Wavelengths for carbon monoxide detection are 1568, 2330 and 4610 nm.
Methane/ Hydrocarbons (CH4) is a colorless, odorless gas commonly used as a fuel (ie the main component of natural gas). CH4 is a by product of landfills, mining, farming, biofuels, composting, and the gas supply chain. Methane gas is essential for the formation of methanol (methyl alcohol), which is a key component of alcohol. The wavelength for measuring methane is 1653 nm.
Sulphur Dioxide (SO2) occurs naturally via volcanic activity and is also produced as a by-product of copper extraction and the burning of Fossil fuels such as coal and diesel. It is sulphur dioxide which is responsible for the smell of burnt matches. Hydrogen Sulfide(H2S) and Hydrogen Flouride (HF) are also poisonous gases emitted by volcanoes.
Hydrogen Fluoride (HF) is a colorless gas with a pungent odor that causes eye irritation and throat irritation. Hydrogen Fluoride is used in pharmaceutical and industrial compounds and polymers, especially Teflon.
Hydrogen Sulfide (H2S) is used in several industries and is a by-product of many industrial processes including oil refining, sewage, mining, tanning, wood pulp processing, food processing, craft paper production, and rayon manufacturing. It has a distinct "rotten egg" smell even at low concentration levels. In addition to being harmful to human health as an asphyxiant, hydrogen sulfide is both flammable and explosive.
Nitric Oxide (NO) is a colorless gas with a sharp odor. It is the main component of photochemical smog and occurs as a by-product of tobacco smoke, and propane, diesel and gasoline engine exhaust. It is also used as bleaching agent for rayon and for making. Nitric oxide gas is also used for medical application such as treating respitory failure in premature babies. Once inhaled, it works by relaxing smooth muscle to widen (dilate) blood vessels, especially in the lungs. Wavelengths for nitrogen oxides detection are 1814 nm, 2270 nm, and 2670 nm
Nitrous Oxide (N2O) commonly referred to as "laughing gas" has significant medical uses, especially in surgery and dentistry, for its anaesthetic and pain reducing effects. N2O is an Aerosol propellant used in car racing to allows the engine to burn more fuel by providing more oxygen, and in Whipped cream and cooking spray. While relatively non-toxic, nitrous oxide can cause ill effects on human health, whether through breathing it in or by contact of the liquid with skin or eyes. Additionally, nitrous oxide depletes vitamin B12. Nitrous oxide is absorbed at approximately 5000 and 8000 nm, as well as in the UV over the wavelength range 173-240 nm.
Ozone (O3) Gas Sensors
Ozone (O3) is an inorganic molecule which in its pure form is a pale blue gas with a distinctively pungent smell. Ozone occurs both in the Earth's upper atmosphere where it is beneficial and at ground level where it is dangerous. Bad ozone occurs when pollutants emitted by cars, power plants, chemical plants, and other sources chemically react in the presence of sunlight. As ozone mixes with other pollutants, it takes a form which is often referred to as ‘Smog’. When inhaled, ozone can damage the lungs and relatively low amounts of ozone can cause chest pain, coughing, shortness of breath and throat irritation. Ozone is most effective at absorbing radiation at the 250 nm wavelength However there are IR wavelengths between 9-10 micron that are also effective
Amonia NH3 is a colorless, alkaline gas comprised of nitrogen and hydrogen (NH3) that has a strong odor, often associated with window cleaner. The main usage(nearly 80%) is in agriculture as fertilizer. Ammonia is also used for refrigerant, water purification, and in the manufacture of plastics, pesticides, dyes and other chemicals. Ammonia is very caustic, and can irritate or burn skin upon contact, damage respiratory systems, and impact vision. Though there are many wavelengths for detection of NH3, 1512 and 3000 nm are often used.
NDIR Sensors
NDIR sensors are typically thermopile sensors. The sensor generate an output (voltage or current) that is proportional to the amount of light (heat) that reaches the sensor. Sensors are available with very small active areas of approx .36 mm² however most NDIR sensors use a larger area of 2 mm² or even 4 mm² areas. Packages are available in SMD to small TO-46 and TO-39.

Chamber:
The best way to maximize the signal reaching the thermopile detector is to use a sample chamber with high reflective properties, which ensures that the detector absorbs the radiation emitted from the source and not the chamber itself. Most chambers are cylindrical though in recent years domed shaped chambers have been implements to increase the path length while reducing the over all length of the systems design. Using a reflective chamber to reduce the amount of radiation absorbed by the chamber can also reduce help to reduce the amount of power consumed by the system because a less powerful radiation source can be used.
NDIR Lamps
NDIR lamps typically range in size from T ¾, T-1, T-1½ and T-1¾ . The "T" indicates the bulb is tubular shaped, while the # "1" denotes the eights of an inch (ie. T-1 is ONE eighths of an inch in diameter.)
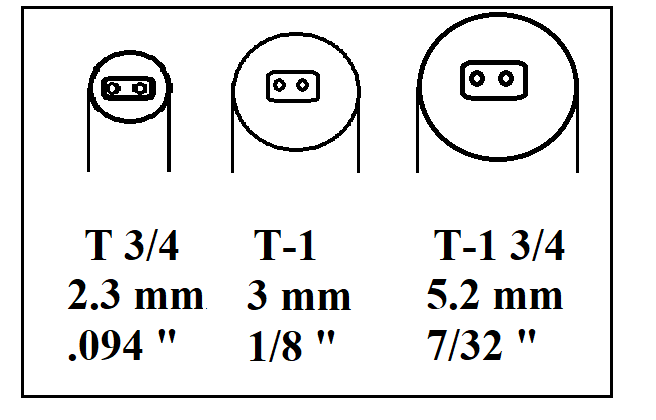
Once the diameter is selected, there are also many base and format to choose from including unbased, based and molded SMD based, wire or bi pin leads and standard or short envelope designs. The image below shows some of the more common sizes and designs.
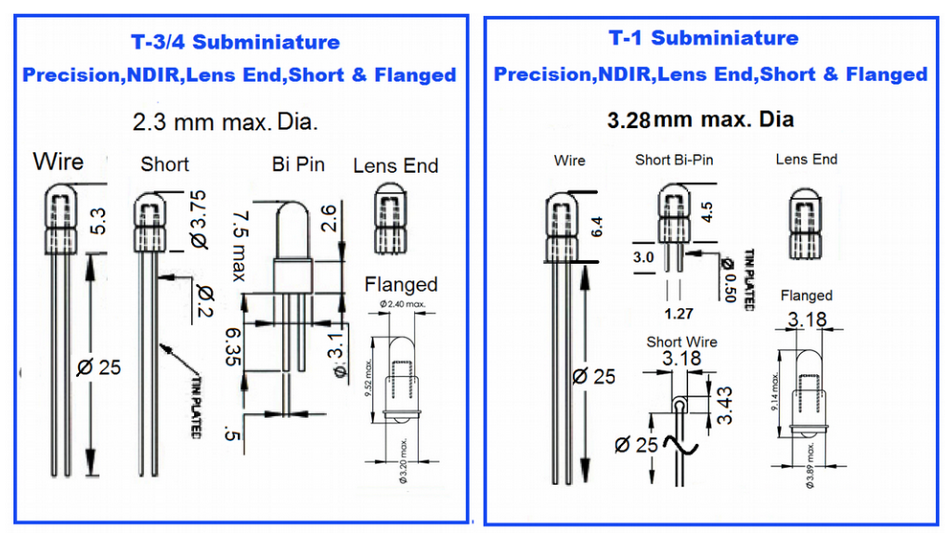
NDIR lamps are typically vacuum lamps which means they do not use a gas such as halogen or argon. NDIR lamps use a tungsten filament inside quartz tube that is hermetically sealed to allow the tungsten to burn by preventing oxidation from exposure to oxygen or water vapor. Most lamps are run for a couple of hours to burn in and anneal the filament and increase the stability of the lamp. Additional burn in is often applied to lamps that require improved stability as most tungsten lamps experience the highest level of change in the initial period of use.
The filament of an NDIR lamp is made of tungsten coils which and are available in a few configurations including C-6, CC-6, C-2R, C-2F, and CC-2V as shown below.
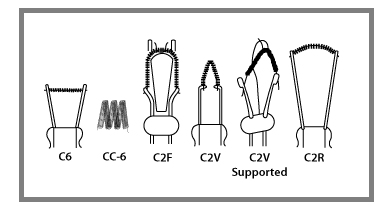
The amount of coils, height and width of coils and coil designs are highly controlled to create the specific features of the lamp including the Voltage, Current, Color temperature, MSCP, and Life hours.
In order to achieved the rated life hours of a lamp, most NDIR gas detector designs include some form of filament protection. A cool unprotected tungsten filament will experience a phenomenon known as In Rush current. This rush of current at start up can be as much as 10 times greater than operating current, leading to strong magnetic forces between adjacent coils of the filament. Inrush current creates both thermal and mechanical stress and is one of the main causes of premature lamp failure. Protection from in rush current may be done with the use of a summer voltage, short period of low current for warm up or protection in the power circuitry.
ILT offers NDIR lamps, MR3 and MR4 Gold, Silver and Aluminum coated parabolic and ellipsoidal reflectors with and without custom focus, as well as custom lamp and reflector designs. We are also able to offer prototype development, samples and final volume assembly of your complete NDIR sensor.
If you would like to speak with one of our experts about which lamp to select for your application, or if you require a custom solution, contact us using the link below.
< Back to All Instrumentation and Sensor Light Sources
View Other Applications
Lamps for Optical Sorting
Polymerase Chain Reaction (PCR)