
One study finds a shocking number of bili blue light therapy systems did not meet the minimum recommended spectral irradiance for intensive PT
For over 60 years, the use of phototherapy (PT) has been used to effectively treat hyperbilirubinemia in term and near-term infants world wide. Neonatal hyperbilirubinemia is common, with half a million or more infants effected by it annually around the globe. If not treated, hyperbilirubinemia in infants can cause a host of lasting disabilities including Kernicterus, encephalopathy, hearing loss, choreoathetoid cerebral palsy, seizures, developmental delay, and other neurological impairments. It is also linked to an increase in the risk of autism.
Light phototherapy is often the first course of treatment for severe, non-genetic or illness-based infant jaundice, reducing the need for transfusions, and often allowing for treatment at a doctors office or at home rather than in a hospital setting. Light therapy is know to greatly improve infant nuerodevelopmental outcomes in the majority of cases.
Current recommendations from the American Academy of Pediatrics state that intensive PT should have at least 30 µW/cm2/nm in the bandwidth of 460 – 490 nm delivered to as much of the body surface area as possible.
Recent studies that looked for variations in the irradiance of phototherapy devices, both domestically and abroad, found a shocking number of devices did not meet the minimum recommended spectral irradiance for intensive PT. A number of factors can contribute to the deficiency including the age and condition of the light source, distance from neonate, other equipment hindering exposure to the light such as ventilators, among other things.
Why Measuring PT Light Sources Is Important
Lamp Aging - All light sources age. With aging comes a decrease in the lamp’s intensity (the amount of light it emits) and, in some cases, a shift in its spectral output. Other environmental factors such as build-up on reflectors, diffuser and lenses or instability in a lamps power supply can also impact a lamp’s performance. Additionally different light sources age differently, eg LED versus fluorescent or Halogen bulbs.
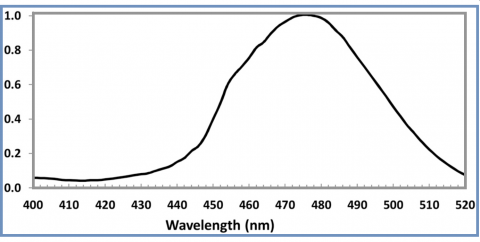
Changing Guidelines and Specifications - The recommended guidelines for spectral irradiance have changed in recent years. Today’s guidelines from the American Academy of Pediatrics specify at least 30 µW/cm2/nm in the bandwidth of 460 – 490 nm. Previous guidelines recommended a broader range from 430 nm to 490 nm, which is still used by many manufacturers and practitioners. Still others recommend alternative ranges such as average irradiance over the range of 420 nm to 500 nm. A recent study by NIH confirmed that for equal irradiance, a fluorescent light source peaked nearer to 490 nm is significantly more effective than one peaked near 450 nm, and that use of LED’s with a peak near 476 nm would be the most efficient for phototherapy, reducing the heat burden of the infant by avoiding absorption of useless light.
Differing Regional Standards – European manufacturers of blue light phototherapy devices are required to test their devices in accordance with the IEC60601-2-50 standard, where in the US no such standard exists. Additionally some European countries recommend measuring the irradiance of phototherapy lamps prior to each treatment. In the US, many hospitals and healthcare facilities have their own protocols and practices for phototherapy.
Differences in Measurement Systems
There are two different types of devices that can be used to measure the blue lights from phototherapy systems used to treat hyperbilirubinemia.
Spectroradiometers – A spectroradiometer measures both the light’s intensity and spectral distribution, e.g. the wavelengths of light being emitted. Spectroradiometers can be beneficial to use on light sources that tend to shift with age, however, these devices can be more complicated to use and the data may require more expertise to analyze the results, in addition to being more expensive to purchase.
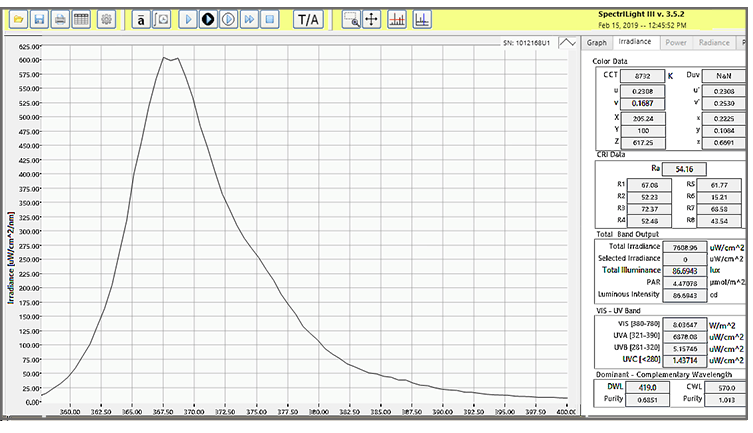
Blue range filtered radiometers - Light meters measure the light levels (irradiance or band irradiance- W/cm2 or uW/cm2/nm) that will reach the patients skin when placed at the functional working distance. A complete system typically consists of a meter and sensor, filtered and calibrated to detect light within the blue range at a specific peak. Because of the varying properties between light sources and manufacturers, some will have proprietary measuring devices calibrated to their specifications. The selection of the meter/sensor with proper blue light filtration to match the light source can some times be challenging as some light source and light meter manufacturers may test different wavelengths in the ~420-520 nm range with peaks varying from 450-490 nm.
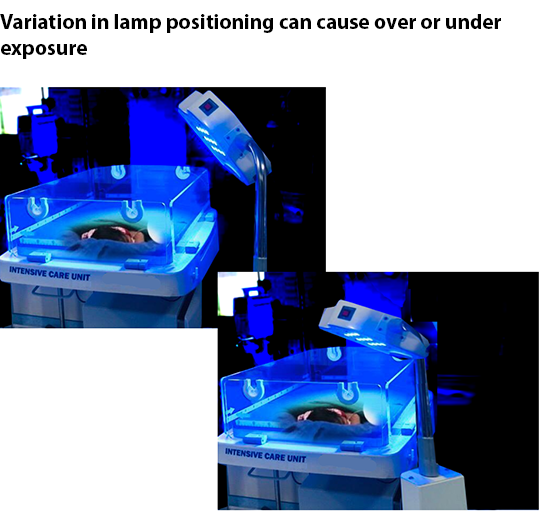
Challenge of Taking Measurements in a Busy NICU
Regardless of the type of light measurement device used, taking measurements of a light pad or light source can be cumbersome and time consuming – something that is not conducive to a busy NICU. Most measurement systems require multiple point-by-point measurements to account for the large area of exposure over the patients skin surface. These measurement must then be added up and divided by the amount of test points to get an average irradiance. Depending on the type of light system, this can be six, ten, or even more individual points of measurement that need to be written down and then tabulated. This process alone can take three to four minutes, and requires the need for a pad of paper, pencil, and calculator.
Researchers looking at variations between phototherapy lamp validation practices among area hospitals noted testing and validation was often impractical due to lack of simplicity and time constraints often delaying on-site measurements. Failure to test or validate the required 30 uW/cm2/nm recommended by the AAP increases the risk of incorrect exposure levels as lamp output, setup and working distances change.
One measurement device aims to take many of those challenges off the table. The ILT750 has been designed to seamlessly take measurements while automatically calculating the average irradiance in a matter of seconds. Simply scan the detector head under the light source or over a bili-pad and let the meter do the rest! Watch our demonstration video below.
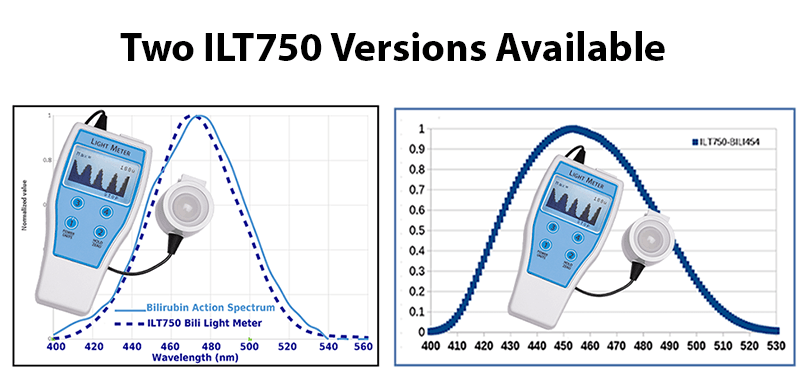
The ILT750 is available in two different configurations to allow comparison to historical products or to the updated specifications.
The ILT750-Bili has a peak response at 470 nm and incorporates ILT’s proprietary BAS filter to more accurately match the bilirubin action spectrum. This meter is ideal for measuring a wide range of light sources including traditional fluorescent, halogen and LED.
The ILT750-Bili454 is designed to be a direct replacement for the now-discontinued GE Ohmeda meter. If you are currently using any of the GE bili systems including the Giraffe, Lullaby, Bilisoft and other traditional GE blue light systems, this meter will read within a few percent of the Ohmeda. The meter is calibrated at 454 nm using a GE LED light source and applies proper band irradiance calculations.
Both models come with an ISO/IEC 17025:2017 accredited calibration certificate, and both are classified as Class 1 medical devices.
References: Variation in the Phototherapy Practices and Irradiance of Devices in a Major Metropolitan Area

