What is Light?
Electromagnetic Wave Theory
Light is just one portion of the various electromagnetic waves flying through space. The electromagnetic spectrum covers an extremely broad range, from radio waves with wavelengths of a meter or more, down to x-rays with wavelengths of less than a billionth of a meter. Optical radiation lies between radio waves and x-rays on the spectrum, exhibiting a unique mix of ray, wave, and quantum properties.
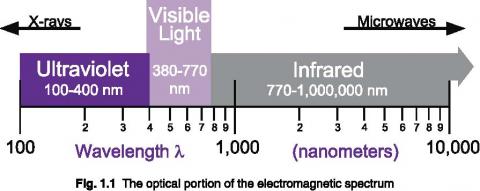
At x-ray and shorter wavelengths, electromagnetic radiation tends to be quite particle like in its behavior, whereas toward the long wavelength end of the spectrum the behavior is mostly wavelike. The visible portion occupies an intermediate position, exhibiting both wave and particle properties in varying degrees.
Like all electromagnetic waves, light waves can interfere with each other, become directionally polarized, and bend slightly when passing an edge. These properties allow light to be filtered by wavelength or amplified coherently as in a laser.
In radiometry, light’s propagating wavefront is modeled as a ray traveling in a straight line. Lenses and mirrors redirect these rays along predictable paths. Wave effects are insignificant in an incoherent, large scale optical system because the light waves are randomly distributed and there are plenty of photons.
Ultraviolet Light
Short wavelength UV light exhibits more quantum properties than its visible and infrared counterparts. Ultraviolet light is arbitrarily broken down into three bands, according to its anecdotal effects.
UV-A is the least harmful and most commonly found type of UV light, because it has the least energy. UV-A light is often called black light, and is used for its relative harmlessness and its ability to cause fluorescent materials to emit visible light - thus appearing to glow in the dark. Most phototherapy and tanning booths use UV-A lamps.
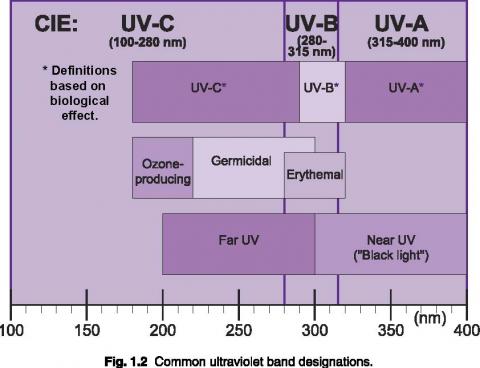
UV-B is typically the most destructive form of UV light, because it has enough energy to damage biological tissues, yet not quite enough to be completely absorbed by the atmosphere. UV-B is known to cause skin cancer. Since most of the extraterrestrial UV-B light is blocked by the atmosphere, a small change in the ozone layer could dramatically increase the danger of skin cancer.
Short wavelength UV-C is almost completely absorbed in air within a few hundred meters. When UV-C photons collide with oxygen atoms, the energy exchange causes the formation of ozone. UV-C is almost never observed in nature, since it is absorbed so quickly. Germicidal UV-C lamps are often used to purify air and water, because of their ability to kill bacteria.
Visible Light
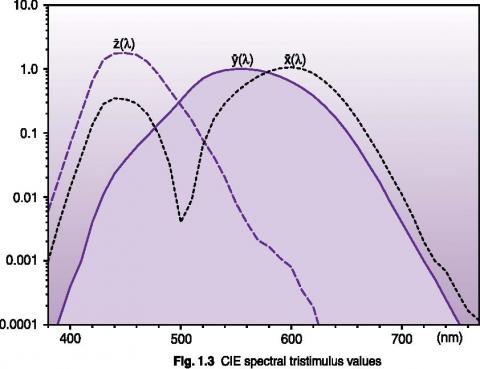
Photometry is concerned with the measurement of optical radiation as it is perceived by the human eye. The CIE 1931 Standard Observer established a standard based on the average human eye response under normal illumination with a 2° field of view. The tristimulus values graphed below represent an attempt to describe human color recognition using three sensitivity curves. The y(λ) curve is identical to the CIE V(λ) photopic vision function. Using three tristimulus measurements, any color can be fully described.
Color Models
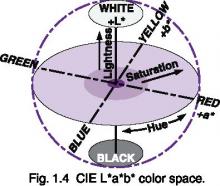
Most models of perceived color contain three components: hue, saturation, and lightness. In the CIE L*a*b* model, color is modeled as a sphere, with lightness comprising the linear transform from white to black, and hues modeled as opposing pairs, with saturation being the distance from the lightness axis.
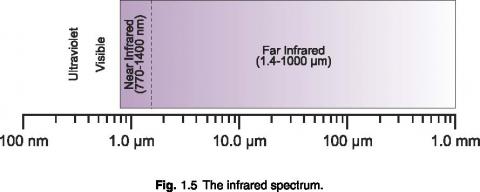
Infrared Light
Infrared light contains the least amount of energy per photon of any other band. Because of this, an infrared photon often lacks the energy required to pass the detection threshold of a quantum detector. Infrared is usually measured using a thermal detector such as a thermopile, which measures temperature change due to absorbed energy.
]
While these thermal detectors have a very flat spectral responsivity, they suffer from temperature sensitivity, and usually must be artificially cooled. Another strategy employed by thermal detectors is to modulate incident light with a chopper. This allows the detector to measure differentially between the dark (zero) and light states.
Quantum type detectors are often used in the near infrared, especially below 1100 nm. Specialized detectors such as InGaAs offer excellent responsivity from 850 to 1700 nm. Typical silicon photodiodes are not sensitive above 1100 nm. These types of detectors are typically employed to measure a known artificial near-IR source without including long wavelength background ambient.
Since heat is a form of infrared light, far infrared detectors are sensitive to environmental changes - such as a person moving in the field of view. Night vision equipment takes advantage of this effect, amplifying infrared to distinguish people and machinery that are concealed in the darkness.
Infrared is unique in that it exhibits primarily wave properties. This can make it much more difficult to manipulate than ultraviolet and visible light. Infrared is more difficult to focus with lenses, refracts less, diffracts more, and is difficult to diffuse. Most radiometric IR measurements are made without lenses, filters, or diffusers, relying on just the bare detector to measure incident irradiance.

Request Light Management Handbook as PDF (ALL Chapters)
Chapter 1 - The Light Measurement Handbook
Copyright © 1997 by Alexander D. Ryer
All Rights Reserved.
No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the copyright owner. Requests should be made through the publisher.
Technical Publications Dept.
International Light Technologies
10 Technology Drive
Peabody, MA 01960
ISBN 0-9658356-9-3
Library of Congress Catalog Card Number: 97-93677
Explore more light measurement resources
