SteriLumen relies on the ILT2400 UVGI as a critical verification and validation tool in their R&D efforts

SteriLumen was founded in 2016 with the goal of helping hospitals combat HAIs (healthcare associated infections) with their patented UVC-based disinfection solutions. Each year millions of people contract an infectious disease from a hospital, assisted living, long-term care facility, or other healthcare environment; and nearly 100,000 die. These infections cost the healthcare industry billions in lost reimbursements and fines each year. With the onset of the pandemic, these facilities quickly started seeing their profits slide into the red.
At its founding SteriLumen focused its efforts on disinfection of high-touch and high-traffic surfaces and sink drains. These areas are particularly susceptible to harboring germs and bacteria that can be easily transferred from one person to the next. Manually cleaning these surfaces multiple times a day is neither practical nor as efficient as a disinfection system that is always working.
For disinfecting high-touch surfaces, SteriLumen launched its Lumicide Ribbon, a connected, configurable platform that can be seamlessly integrated into new spaces, or retrofitted into existing ones. The Lumicide Ribbon system is designed to always be on, providing maximum germicidal irradiation to the surface it’s disinfecting. Since UVC light is harmful to people, the Lumicide system has a dual, redundant motion sensor integrated into it ensuring that the system is switched off when someone approaches. We spoke with Don Simmons, Chief Engineer at SteriLumen, to find out why they selected the ILT2400 UVGI Light Measurement System.
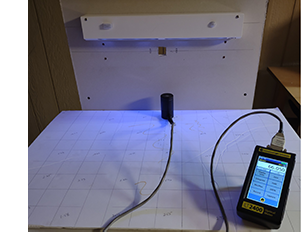
For Don, the selection of the ILT2400 was multi-fold. Prior to purchasing the radiometer, SteriLumen had been relying on software modeling to predict where the UV light was going, and at what threshold a person would be exposed to UV hazard when the device was on. On top of that, they were relying on outside testing services to validate that their system was emitting the proper amount of light, or dose, to effectively inactivate germs. These tests are expensive and time consuming, and not conducive to iterative development, ongoing improvement, and tight time lines.
“I had always worked places that had testing and validation tools on-site, and I knew if we were going to effectively meet our engineering objectives, we would need that capability here as well,” explains Don. “The addition of the ILT2400 enabled us to pull that capability in-house, saving us an enormous amount of time and resources in outside testing services,” continued Don.
With the onset of Covid the market has been flooded with all kinds of UVC devices making all kinds of claims regarding disinfection. For SteriLumen they back their claims up with data – and lots of it. Often going beyond the regulatory requirements, SteriLumen was looking to show that their system was both effective at inactivating pathogens, and safe from being a UV hazard. SteriLumen’s Lumicide systems have been UL tested for efficacy and are currently being investigated for adherence to UL8802.
Don explained “When we received the data back from UL, our numbers were almost an exact match. There were no surprises. This gave us the confidence we would pass
biological efficacy testing, which costs many thousands of dollars more, before we sent the system in.” SteriLumen makes their independently conducted efficacy tests available upon request.
The ILT2400 not only helped SteriLumen with their development and regulatory and efficacy test preparation, it also enabled them to effectively evaluate new components from potential suppliers. As Don puts it, “We would receive in chips from all kinds of suppliers, claiming all kinds of things. We had to rely on their specifications without being able to verify them, and for us, that just wasn’t good enough.” We asked Don why he ultimately chose ILT for his meter. “Simple,” he said. “I was already familiar with ILT having used your equipment previously, you’re a local company, and you have great tech support.”
Since buying their ILT2400 meter, SteriLumen also acquired Akida LLC, makers of the Airocide product line, to address air-borne pathogens such as coronavirus. “This system,” Don explains, “is very different from the Lumicide. It uses a totally different light engine, with totally different properties. I’m just starting to get my arms around this system from an engineering perspective, but what’s been helpful is that with the addition of a second detector calibrated for that source type, the ILT2400 can validate both.”
About SteriLumen
SteriLumen develops Data Driven Disinfection™ systems that are connected, configurable, and validated. Their Lumicide product is a configurable system for disinfecting high touch surfaces. Their Airocide system has FDA class II medical device clearance for air disinfection. Their Clarity 3D connected monitoring delivers Data Driven Disinfection™ with the ability to schedule and modify disinfection cycles and get reporting and maintenance alerts, and setup and program new devices from a browser or mobile device. SteriLumen’s most recent efficacy test of the Lumicide Ribbon unit this past June, resulted in SARS-CoV-2 viral inactivation (i.e., viral particles rendered non-infectious) >3.04-log reduction (>99.908%) at the 5-minute exposure time and >3.66-log reduction (>99.978%) at the 20-minute exposure time.
It has also been tested on C. diff, E.coli, MRSA and H1N1 with similar viral inactivation rates. You can learn more about SteriLumen’s products at their website
here.
Download PDF version of this profile
*Efficacy data is the sole ownership of SteriLumen.
© International Light Technologies | 2021
Data Driven Disinfection and Lumicide, are registered trademark of SteriLumen.
All trademarks are the property of their respective owners.

